About Project
Transforming Cancer Research
Our goal is to develop innovative 3D in vitro tumor models to advance cancer research and drug testing, utilizing cutting-edge biotechnologies and interdisciplinary collaboration.
BioengineeredTumor

The aim of the project “Biomimetic tumor engineering to enhance drug discovery – BioengineeredTumor” is to contribute to advancements in cancer research and drug testing. Developing anti-cancer drugs is a slow process due to limitations in current preclinical tests and low correlation between these results and clinical trials. To tackle these challenges, a team of engineers from the Faculty of Technology and Metallurgy (TMF) and the Innovation Center of FTM (ICTMF), along with life scientists from the Institute of Molecular Genetics and Genetic Engineering (IMGGI), the Faculty of Medicine, the Institute for Biological Research “Siniša Stanković” (IBIS) and the Faculty of Pharmacy, all at the University of Belgrade, aims to create new, reliable, and efficient 3 dimensional (3D) in vitro tumor models. These models are designed to be simple, reliable, and effective for testing of anti-cancer drugs. The approach involves using various human and animal cell lines of malignant tumors together with alginate-based biomaterials as artificial extracellular matrices and perfusion bioreactors to simulate the tumor environment. The ultimate goal is to establish a platform for long-term in vitro cultures of malignant tumor cells aimed for applications in discovery and validation of anti-cancer drugs, development of personalized medical treatments, and advancements of cancer research.
The project has started on January 1, 2024 with the budget of € 281 k.
Objectives
The overall objective of the project is to develop novel, simple and robust longer-term 3D in vitro tumor models for reliable anti-cancer drug testing by applying biomimetic principles to tumor engineering.
Specific objectives of the Project are:
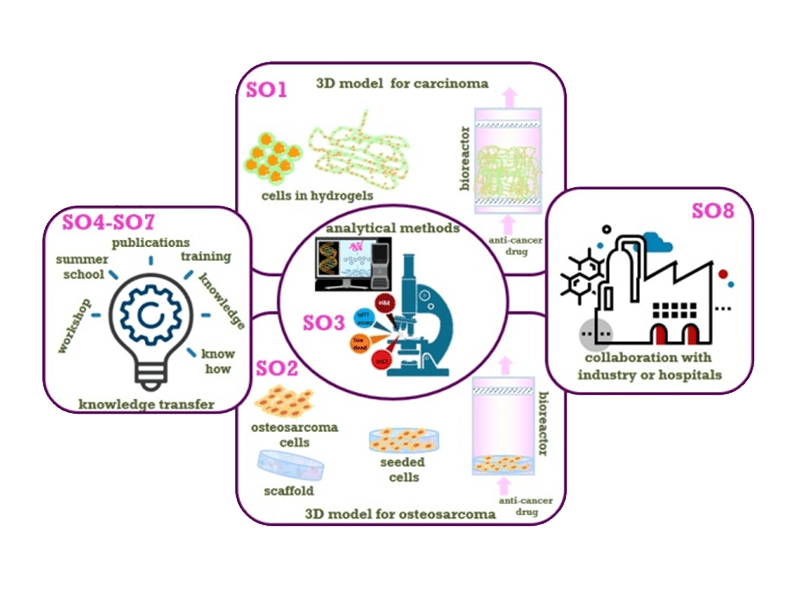
Develop at least one 3D culture model system for carcinomas and validate it in short-term studies (up to 7 days) and a longer term study (up to 28 days) using standard anti-cancer drugs.
Develop at least one 3D culture model system for osteosarcoma cells and validate it in short-term studies (up to 7 days) and in a longer term study (up to 28 days) using standard anti-cancer drugs.
Develop or adapt analytical methods for reliable assessment of the viable cell density and distribution within alginate-based supports.
Gain knowledge in cancer cell biology.
Disseminate the results by publication of manuscripts in peer-reviewed scientific journals and presentations at national and international scientific conferences.
Train five ESRs in cancer cell cultures within the project.
Organize one workshop and one summer school on 3D tumor models.
Establish a collaborative project with industry or end-user (e.g. hospital) addressing development of anti-cancer drugs, cancer cell culture or cancer research.

Concept and methodology
The overall concept of the project addresses the in vitro–in vivo gap between the data obtained in 2D monolayer cell cultures and those obtained in vivo either in animal studies or in human clinical trials, with respect to cancer research and anti-cancer drug discovery, in particular. 3D in vitro culture models have been recognized as a tool that could bridge the in vitro-in vivo gap, decrease the need for animal experimentation, and provide more reliable in vitro models. One of the approaches to create such models is tumor engineering, which uses the strategy of tissue engineering. In this concept, biomaterial scaffolds are used to provide a suitable 3D environment to cancer cells, while bioreactors should provide efficient mass transport to the cultivated cells within the scaffold at adequate hydrodynamic conditions.
The first concept point is that development of a longer-term in vitro 3D tumor model relies on the use of biomimetic scaffolds and bioreactors providing adequate biochemical signals.
The second concept point is that bottom-up approach starting form single well-defined components can yield controlled and reproducible 3D tumor models recapitulating some of the main physiological aspects of cancer cells in vivo.
The third concept point is that simple in vitro 3D tumor models capturing some of the key features of the tumor environment in vivo amended with appropriate techniques to analyze the cultured cells can present a useful and expandable platform for reliable anti-cancer drug testing and cancer research.
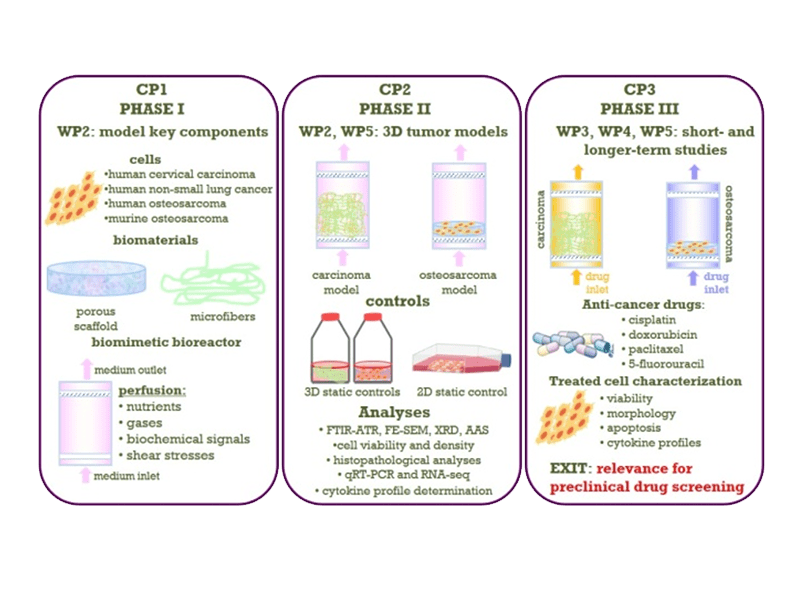
The methodology applied in the proposed project is designed according to the concept points comprising 3 phases: I) development of 3D tumor models, II) validation of the 3D tumor models, and III) utilization of the optimized 3D tumor models in short- and longer-term studies of the effects of standard anti-cancer drugs.
Work Packages
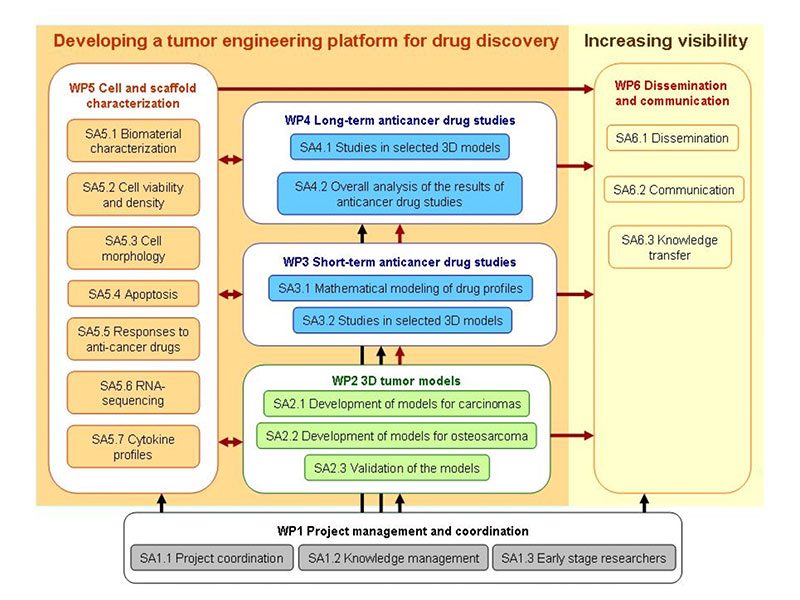
The project is divided into 6 work packages (WP):
- The WP1 (Project management and coordination) is allocated to overall project management and coordination.
- The WP2 (3D tumor models) aims at establishing 3D models for cultures of carcinoma and of osteosarcoma cells as a platform for cancer research and preclinical pharmacological studies. It comprises production and characterization of several novel bioactive scaffolds, development of cell immobilization protocols, utilization of perfusion bioreactor for maintenance of a longer-term cultures and evaluation of cultivation parameters on the cultured cells to yield optimized 3D carcinoma and osteosarcoma models as the major outcome of this WP.
- The WP3 (Short-term anti-cancer drug studies) presents evaluation of the 3D culture platform developed in WP3 for potential utilization in anti-cancer drug screening. Therefore, this WP comprises short-term studies of the effects of standard anti-cancer drugs applied in the optimized 3D carcinoma and osteosarcoma models.
- The WP4 (Long-term anti-cancer drug studies) presents evaluation of the 3D culture platform developed in WP3 for potential utilization as a preclinical pharmacological tool for validation of anti-cancer drugs applied in clinically relevant regimens. The outcome of this WP will be guidelines for utilization of the platform as well as for further adaptation to other cell cultures.
- The WP5 (Analytical methods) comprises all analytical methods and techniques that will be used in the project for comprehensive characterization of biomaterials used as well as cultured cells in 3D environments. It is also expected that some novel and modified techniques will be developed for characterization of cells within biomaterial matrices.
- The WP6 (Dissemination and communication) is allocated to dissemination and promotion of the results during the lifetime of the project and afterwards.

Mission
Our mission is to develop an effective yet sufficiently simple platform for 3D cultures of cancer cells suited for the use by scientists without technical expertise. In specific, by applying a systematic and integrated methodology, we aim to develop 2 novel 3D models for cultures of carcinoma and osteosarcoma cells by comprehensively defining the key model components in conjunction with developing/adapting analytical methods for reliable and reproducible characterization of cells within 3D scaffolds. We will validate the models in short- and longer-term cell cultures by application of standard anti-cancer drugs in clinically relevant regimens with the ultimate goal to demonstrate potentials of the developed platform for use in anticancer drug testing.
Vision
Our vision is to establish a systematic methodology based on defining key parameters for development of 3D in vitro cancer models by gradual building the complexity towards the in vivo-like tumor features. The ultimate vision is adaptation of the BioengineeredTumor approach and the platform to various cell types as well as autologous cells of patients for utilization in designing personalized therapies.

Science Fund of the Republic of Serbia under the grant no. 7503.
BioengineeredTumor © 2024 | Web Design: Strumark